Polymer Chemistry
Materials Chemistry, Surface and Colloidal Science, and Environmental Chemistry
Our research program is focused on rational design and precise synthesis of responsive and functional polymers for advancing fundamental understanding, exploring potential applications, and addressing technological challenges. Current research interests include: (i) macromolecular brush materials (polymer brushes, hairy particles/brush particles, and bottlebrush polymers), (ii) stimuli-responsive polymers (nonionic, ionic, and zwitterionic), (iii) polymer materials for removal of toxic ions from water (polymer brushes and covalent networks), and (iv) ferroelectric liquid crystalline polymers (mesogen-free and fluorine-free). “Living”/controlled polymerization methods, such as atom transfer radical polymerization, reversible addition-fragmentation chain transfer polymerization, ring-opening polymerization, ring-opening metathesis polymerization, etc., are used to prepare well-defined polymers. Various analytical methods, including electron microscopy, confocal imaging, dynamic light scattering, pendant drop tensiometry, rheology, differential scanning calorimetry, and isothermal titration calorimetry, are applied to investigate the structures and properties of the synthesized polymers. Our research involves polymer science, materials chemistry, surface and colloidal science, and environmental chemistry. Potential applications in high-performance emulsifiers, substance delivery, water treatment, energy efficiency improvement, and energy conversion are being pursued.
1. Macromolecular Brush Materials
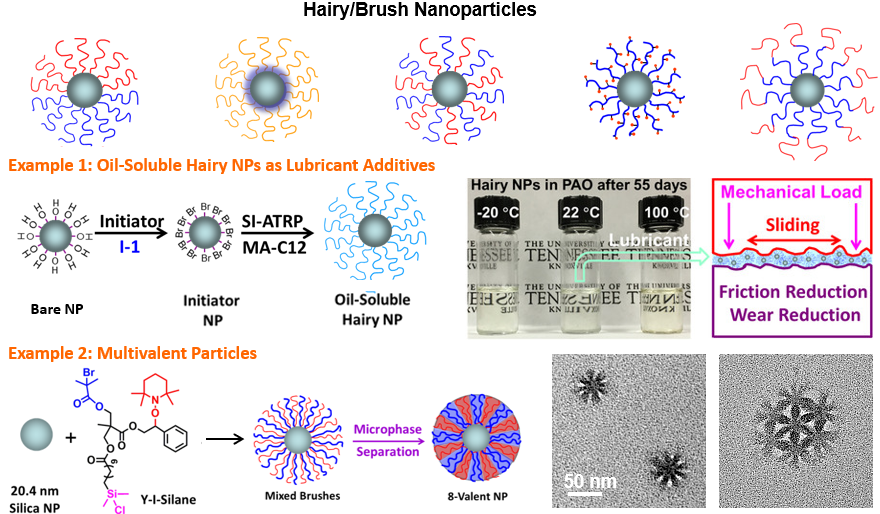
Macromolecular brush materials are featured by densely grafted polymer chains, either on a flat solid surface (commonly called polymer brushes), or (nano)particles (hairy or brush particles), or a polymer backbone (molecular bottlebrushes or bottlebrush polymers). In all these systems, polymer chains are stretched due to steric hindrance, exhibiting deformed conformations under equilibrium conditions. The intriguing structures and properties of macromolecular brush materials have attracted tremendous attention in the past years. We are especially interested in the synthesis, behaviors, and applications of well-defined polymer brush-grafted nanoparticles and multicomponent bottlebrush polymers. Current projects include: (i) new strategies for synthesizing well-defined hairy particles with unique brush architectures, (ii) self-assembly behavior of multicomponent brush particles in solution, at interfaces, and in bulk, (iii) stimuli-responsive molecular bottlebrush emulsifiers, and (iv) applications of macromolecular brush materials in oil lubrication, polymer nanocomposites, and encapsulation and delivery of substances.
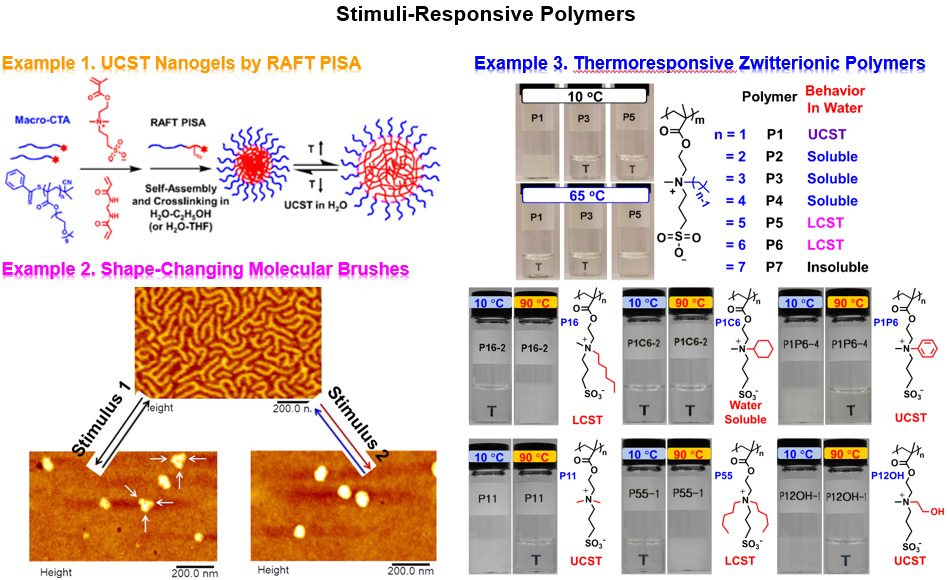
2. Stimuli-Responsive Polymers
Stimuli-responsive polymers undergo large and abrupt physical or chemical changes, often accompanied with a drastic solubility variation in solvents, in response to external stimuli. The changes can be reversible or irreversible, and various stimuli-responsive (temperature, pH, light, specific species, and mechanical force) polymers have been reported. We are especially interested in: (i) thermoresponsive zwitterionic polymers that display lower critical solution temperature (LCST) and upper critical solution temperature (UCST) transitions in water, (ii) zwitterionic hairy nanogels and microgels, (iii) specific ion-responsive polymers, and (iv) stimuli-responsive shape-changing bottlebrush polymers. The following shows examples of UCST thermoresponsive hairy nanogels, stimuli-responsive shape-changing molecular bottlebrushes, LCST and UCST thermoresponsive zwitterionic polymers.
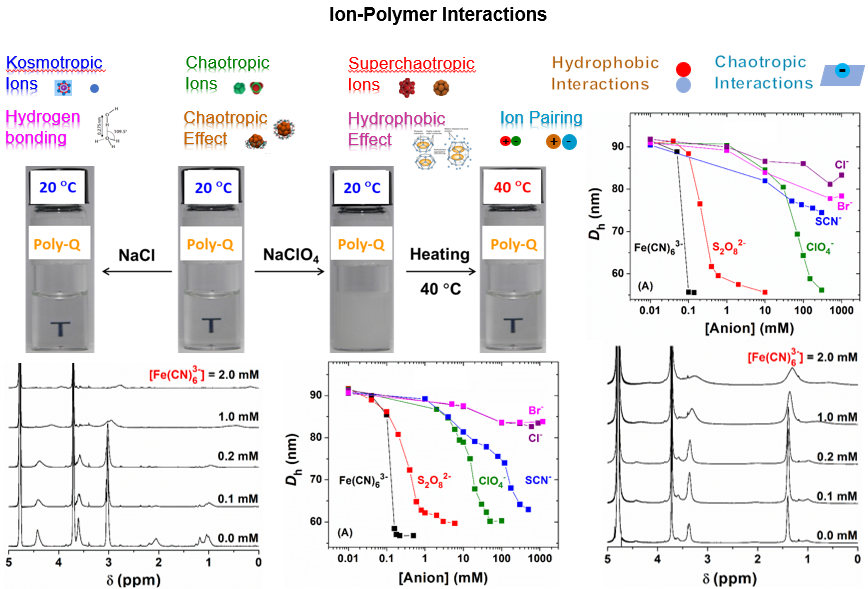
3. Polymer Materials for Water Treatment
Oxyanions are polyatomic oxygen-containing negatively charged ions with a relatively large size, many of which, e.g., perchlorate, arsenate, tellurate, pertechnetate, perrhenate, etc., are toxic even at trace levels, highly persistent, and extremely mobile in aqueous environments. For example, in considering the health risk of perchlorate, the California Department of Public Health has set a maximum contaminant level of 6 micrograms/L (6 ppb) for perchlorate in drinking water. We are designing and synthesizing highly efficient polymer adsorbents for removal of hazardous oxyanions from water based on our understanding of ion-polymer interactions achieved through systematic studies. Various organic materials, including macromolecular brush materials, microporous polymers, and covalent polymer networks, will be prepared and investigated. In addition, we are developing analytical methods to detect trace amounts of specific oxyanions in aqueous solutions.
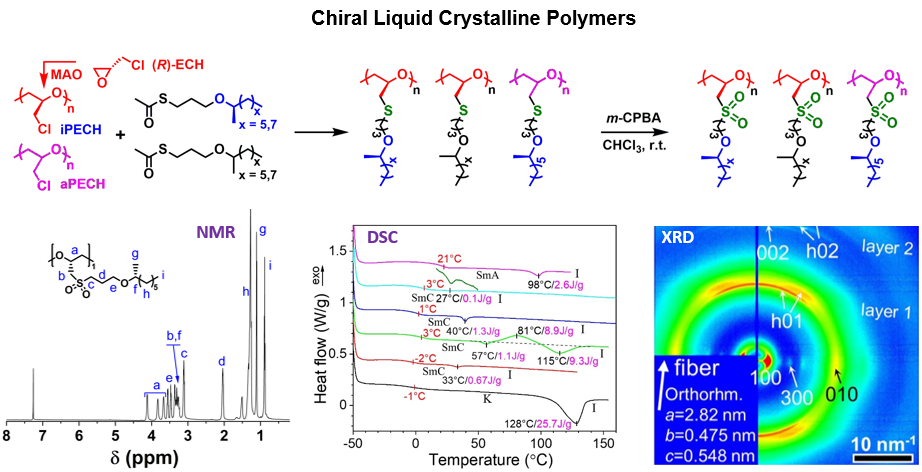
4. Mesogen-Free Liquid Crystalline Polymers
Chiral liquid crystalline polymers (LCPs) hold potential in electronic and electrooptical applications. However, traditional chiral smectic C (SmC*) LCPs display rather small spontaneous polarization (Ps) due to the use of bulky aromatic mesogens and weak dipolar groups, which has effectively hindered the practical use of ferroelectric LCPs. To seek large Ps, we are designing and synthesizing mesogen-free LCPs in collaboration with Professor Lei Zhu of Case Western Reserve University by incorporating highly dipolar sulfonyl groups into the alkyl side chains of polyethers. To induce the formation of SmC* phases, we introduce chiral centers into either the polymer backbone or aliphatic side chains or both. Ring-opening polymerization is useed to prepare comb-like mesogen-free LCPs along with post-polymerization modification reactions. These polymers are characterized by 1H and 13C NMR spectroscopy, size exclusion chromatography, polarimetry, and thermogravimetric analysis, and their liquid crystalline behavior and structures are investigated by differential scanning calorimetry, synchrotron X-ray diffraction, and polarized light microscopy.